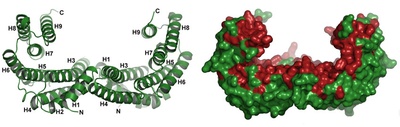
14-3-3 proteins
14-3-3 proteins are a family of regulatory molecules, which specifically bind to phosphoserine (or phosphothreonine)- containing motifs (pSer/pThr) in a sequence-specific manner. Through these binding interactions, 14-3-3 proteins play key regulatory roles in signal transduction, cell cycle control, metabolism control and apoptosis. More than 200 14-3-3 binding partners have been reported so far and some of them play prominent roles in cancer development (e.g. transcription factors p53 and FOXO), neurodegeneration (e.g. Tau protein, ASK1 kinase), cardiovascular diseases (e.g. RGS proteins, phosducin) or inflammation (e.g. NFkB, ASK1 kinase). Mechanistically, 14-3-3 proteins act as allosteric regulators and/or molecular scaffolds that constrain the conformation of the binding partner; if the target protein is an enzyme, this can affect its catalytic activity. Nonetheless, the underlying molecular mechanisms are only partially identified, mainly due to the lack of structural data.