Neutral trehalase Nth1
Neutral trehalase 1 (Nth1) catalyses the hydrolysis of trehalose to glucose. Trehalose is found in many organisms ranging from bacteria to higher plants, and in yeast it functions as a major storage carbohydrate, carbon source and a stress protectant. The activity of yeast Nth1 is triggered by the protein 14-3-3, an interaction that is phosphorylation dependent. Main goal of this project is to elucidate the molecular mechanism of the 14-3-3-dependent activation of yeast Nth1. This project was funded by Czech Science Foundation (Project P207/11/0455).
On the picture the crystal structure of the complex between phosphorylated Nth and Bmh1 (yeast 14-3-3 protein) (PDB 5N6N). The protomers of the Bmh1 homodimer are shown in yellow and brown. Nth1 is shown in blue. The phosphorylated Ser60 and Ser83 are shown as sticks. The calcium ion is shown in orange.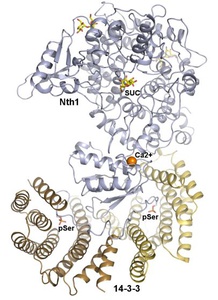
Structural analysis revealed that the binding of phosphorylated Nth1 by 14-3-3 triggers Nth1’s activity by enabling the proper 3D configuration of Nth1’s catalytic and calcium-binding domains relative to each other, thus stabilizing the flexible part of the active site required for catalysis (Alblova et al. (2017) PNAS USA).
Results were published in: Veisova et al. (2012) Biochem. J.; Macakova et al. (2013) Biochim. Biophys. Acta; Kopecka et al. (2014) J. Biol. Chem.; Kopecka et al. (2017) PNAS USA.

