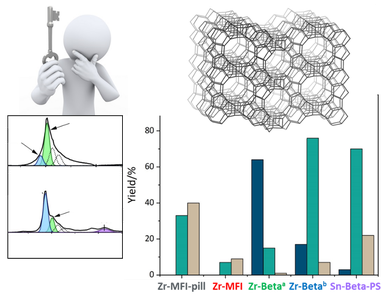
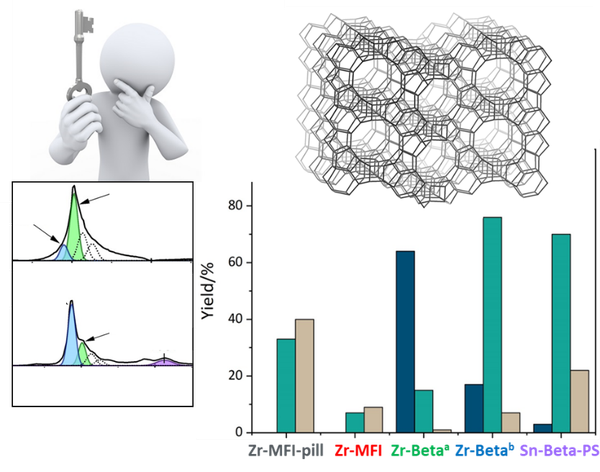
Selectivity driving parameters in redox reactions catalyzed by Lewis acid zeolites.
Project supervisor Ing. Jan Přech, Ph.D.
Zeolites are robust and environmentally friendly heterogeneous catalysts. They contain framework metal ions, which act as Bronsted or Lewis acid sites. In particular, the Lewis acid sites (generated, e.g., by incorporated Ti, Sn, Zr, and Hf ions) catalyze selective redox reactions such as oxidation with peroxides or hydrogen transfer reactions. Type and coordination of the acid sites together with their confinement and surrounding channel environment define the site catalytic properties.
The overarching aim of this PhD project is to find parameters, which define the selectivity in selected redox reactions, use these parameters to drive the selectivity and ultimately induce a stereoselective progress of the reaction.
Publications of the research group relevant to the topic:
From 3D to 2D zeolite catalytic materials
Catalytic performance of advanced titanosilicate selective oxidation catalysts – a review