New publication in Nature Communications
Collaborative theoretical-experimental research between Charles University and St Andrews discovers unexpected facile room-temperature hydrolysis pathways in aluminosilicate zeolites.
Abstract
Aluminosilicate zeolites are currently the most important class of materials for high temperature catalysis, from crude oil processing to exhaust aftertreatment processes. However, despite increased interest in zeolites for low temperature catalysis, including biomass conversion and medicine, an understanding of zeolite framework behaviour under mild conditions is lacking.
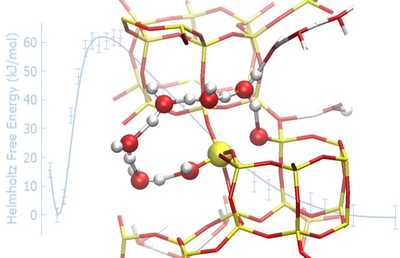
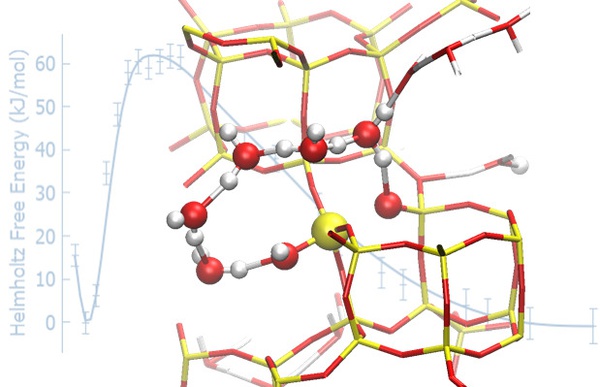
Based on an extensive computational investigation carried at the Faculty of Science, Charles University, within the CUCAM project, we have been able to show that covalent bonds in the zeolite framework are labile when in contact with neutral liquid water, which leads to partial but fully reversible hydrolysis without framework degradation. We also predict novel, energetically viable reaction mechanisms by which Al-O and Si-O bonds rapidly and reversibly break at 300 K. These very unexpected results were confirmed by means of solid-state NMR experiments carried out by collaborators at the University of St Andrews, demonstrating that isotopic substitution of 17O in the zeolitic framework occurs at room temperature in less than one hour of contact with enriched water. The framework is found to heal, with no long-term degradation of the framework over at least 200 days.
The observation that zeolites are dynamic entities under mild aqueous conditions, with fast, reversible breaking of the framework, challenges the conventional view of these materials as static and inert. This works highlights the importance of a proper treatment of temperature and solvation when investigating catalysts, both via theoretical and experimental methods.
Fast room temperature lability of aluminosilicate zeolites