PhD fellow in Sugar and pH-responsive multicompartment nano-assemblies for dual-drug solubilization and delivery
Ing., Ph.D., Mariusz Uchman
Project summary
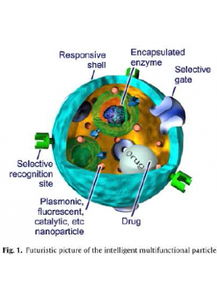
The concept of Multicompartment Micelles (MCMs), i. e., self-assembled aggregates with moieties of different chemical properties, that mimic biological molecules such as proteins was derived by Prof. H. Ringsdorf almost twenty years ago and theoretically described by de Gennes. Figure 1 represents a rather futuristic view of multifunctional smart nanoparticles that possess various sub-domains and functions that coexist and perform in close proximity without mutual interference or with controlled interactions, as it works in nature. The self-assembly process of amphiphilic polymers in water is an attractive platform for preparation of such multi-compartment nanoparticles, due to the mutual incopatibilities of the polymers involved.
The overall goal of this proposal is to gain new information on nanostructure and dynamics of newly designed MCMs based on biodegradable and biocompatible ABC triblock terpolymers. The most often used ABC terpolymers in the research of the MCMs consist of highly incompatible hydrocarbon and fluorocarbon blocks that ensure compartmentalization of nanoparticles, however the lack of responsive functional groups to biologically relevant environment is the main drawback of such MCMs. Much less is known about MCMs that contains hydrocarbon blocks only and boronic acid derivatives. This is why we will focus on synthesis and self-assembly of new ABC triblock terpolymers having at least one boronic acids (bezoxaborol or Wulff-type boronic acids) containing block combined with a hydrophilic and hydrophobic one. By incorporating boronic acids, amphiphilic nanoparticles can be prepared that reversibly bind to 1,2- or 1,3- diols and catechol-containing molecules as well as they are responsive to subtle changes in solution pH (relevant in cancer therapy) and saccharide concentration (relevant for diabetes - related applications). In addition the hydrophobic core forming block will accommodate hydrophobic drugs or fluorophores. The neutral hydrophilic block forms the stabilizing corona of the micelles.
The subdivided core of MCMs serves as a microcontainer for a variety of different active agents. This is why the next goal is to broaden the knowledge on selective solubilization of aromatic compounds (drugs) and their sequential release from MCMs to achieve a synergistic antitumor effect and improve the therapeutic index. Fluorescence techniques will allow us to provide information on local physicochemical properties like micropolarity, microviscosity, local electrostatic potential or accessibility of the fluorophore’s (drugs) neighborhood by certain molecules like quenchers.
Profile of an ideal candidate
Good knowledge of English (FCE equivalent or better)
Background in polymer chemistry and/or phenylboronic acid synthesis and characterization
Experience with scattering techniques, drug solubilisation and delivery
Publications
Glucose-Responsive Hybrid Nanoassennblies in Aqueous Solutions: Ordered Phenylboronic Acid within Intermixed Poly(4-hydroxystyrene)-block-poly(ethylene oxide) Block Copolymer By: Matuszewska, Alicja; Uchman, Mariusz; Adamczyk-Wozniak, Agnieszka; et al. BIOMACROMOLECULES
- Volume: 16 Issue: 12 Pages: 3731-3739 Published: DEC 2015
Preparation of lactic acid- and glucose-responsive poly(epsilon-caprolactone)-b-poly(ethylene oxide) block copolymer micelles using phenylboronic ester as a sensitive block linkage By: Vrbata, David; Uchman, Mariusz NANOSCALE
- Volume: 10 Issue: 18 Pages: 8428-8442 Published: MAY 14 2018
Current research grants of the group
Czech Science Foundation grunt no. 17-00289Y: Sugar and pH-responsive multicompartment nano-assemblies for controlled drug solubilization and release