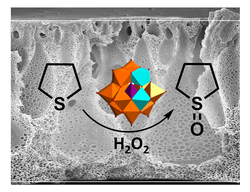
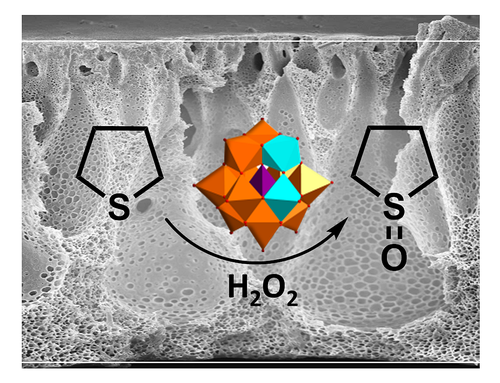
Well-defined copolymers and block copolymers featuring charge-tunable groups in the side chain are ideal materials for the design and modification of interfaces in various settings [1] or for controlling and directing self-assembly processes [2]. We herein report on the synthesis of polyelectrolytes, polyampholytes, and polyzwitterions as well as the corresponding block copolymers using free radical and controlled radical polymerization techniques. One key building block in our setting is polydehydroalanine (PDha),[3,4] a polyzwitterion with high charge density and, depending on the pH, tunable net charge (Figure 1). Apart from interesting solution characteristics [5], we have used PDha and partially protected derivatives as coating materials for iron oxide nanoparticles or within polyelectrolyte multilayers and could show that this allows reversible adsorption / desorption experiments using various oppositely counterparts, including proteins and model pollutants such as methylene blue [6,7]. Besides PDha, we are also interested in the incorporation of polymerizable naphthol derivatives to create polymeric photoacids which show different charge characteristics and solubility in aqueous environment with and without irradiation [8]. Finally, once charge-tunable building blocks are introduced as responsive segment in block copolymer membranes, electrostatic adsorption can be used to immobilize powerful oxidation catalysts such as polyoxometalates based on Mn and V. We could show that such self-supporting hybrid and heterogeneous oxidation catalysts can be used in different model reactions both under static conditions as well as under flow (Figure 2)[9]
References:
1. C. Barner-Kowollik, A. S. Goldmann, F. H. Schacher, Macromolecules 2016, 49, 5001-5016 (Perspective Article).
2. F. H. Schacher, J. C. Brendel, Chem. Asian J. 2018, 13, 230-239.
3. U. Günther, L. V. Sigolaeva, D. V. Pergushov, F. H. Schacher, Macromol. Chem. Phys. 2013, 214, 2202-2212.
4. M. Billing, F. H. Schacher, Macromolecules 2016, 49, 3696-3705.
5. M. Billing, G. Festag, P. Bellstedt, F. H. Schacher, Polym. Chem. 2017, 8, 936-945.
6. M. v. d. Lühe, A. Weidner, S. Dutz, F. H. Schacher, ACS Appl. Nano Mater. 2018, 1, 232-244.
7. P. Biehl, M. V. D. Lühe, F. H. Schacher, Macromol. Rapid Commun. 2018, 39, 1800017
8. F. Wendler, K. Schneider, B. Dietzek, F. H. Schacher, Polym. Chem. 2017, 8, 2959-2971.
9. I. Romanenko, M. Lechner, F. Wendler, C. Hörenz, C. Streb, F. H. Schacher, J. Mater. Chem. A 2017, 5, 15789-15796.
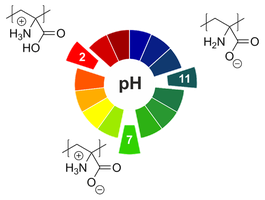
Figure 1: pH-dependent charge characteristics of PDha.
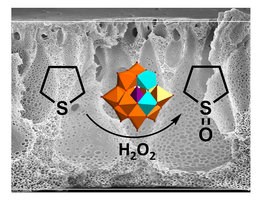
Figure 2: Oxidation of tetrahydrothiophene to the corresponding sulfoxide under flow using immobilized Mn-V-POMs in block copolymer membranes.