Determining Short- and Long-Range Aluminium Ordering in Zeolite Structures
by Dr., Przemyslaw Rzepka
The short-range order of aluminum atoms within periodic zeolite structures critically influences the presence and behavior of catalytically active centers. In Al-rich zeolites (Si/Al<10), Al-O-Si-O-Al sequences stabilize divalent cations or adjacent acid sites. In Si-rich zeolites (Si/Al > 10), Al atoms
emerge as isolated species, Al pairs (Al(-O-Si-O-)1-2Al), or close Al atoms (Al(-O-Si-O-)3Al). Zeolites with a higher fraction of Al pairs exhibit greater catalytic activity. The local organization of Al atoms is influenced by synthesis conditions, offering a powerful means to tailor zeolite structures for optimizing active centers and fine-tuning catalytic properties. However, no universal method currently exists to directly identify the specific T-sites in a zeolite framework occupied by Al atoms.
We developed an anomalous X-ray powder diffraction (AXRD) methodology at the Al K-edge (1.56 keV), where the scattering form factor of Al changes significantly while that of Si remains constant (Fig. 1). These variations in scattering power selectively highlight the presence of Al within the structure. AXRD highlights quantitative information on the long-range structural order of Al:Si distributions, enabling unambiguous identification of Al sites within the framework.
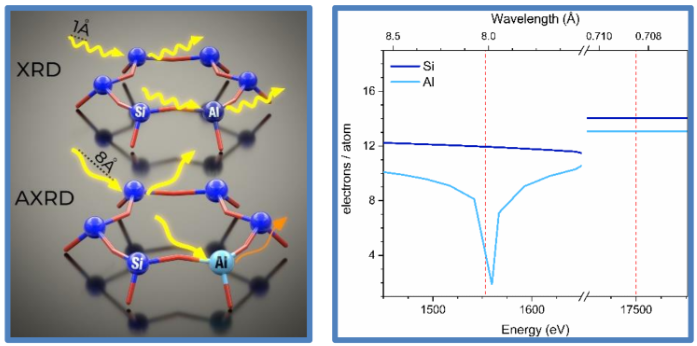
Figure 1. AXRD phenomenon: attenuation of the aluminum signal at the Al K-edge (left). The real part (f0 + f1) of the atomic scattering factor (f = f0 + f1 + i ∙ f2) for silicon and aluminum (right).
The H-ZSM-5 zeolite studied here has a framework structure with 12 T-atoms in the Pnma unit cell, forming a 3D network of intersecting straight and sinusoidal 10-ring channels.
Two samples with similar Si/Al molar ratios (18 and 15) but differing Al pair concentrations (9% and 82%) were analyzed using Co(II) as a probe molecule. Spectroscopic studies previously identified divalent cobalt cations at elongated 6-rings formed by two-folded 5-rings (α site) and at 5- or deformed 6-rings (β site), assigning them to the straight and sinusoidal channel walls, respectively.
The H-ZSM-5 zeolite studied here has a framework structure with 12 T-atoms in the Pnma unit cell, forming a 3D network of intersecting straight and sinusoidal 10-ring channels. Two samples with similar Si/Al molar ratios (18 and 15) but differing Al pair concentrations (9% and 82%) were analyzed using Co(II) as a probe molecule. Spectroscopic studies previously identified divalent cobalt cations at elongated 6-rings formed by two-folded 5-rings (α site) and at 5- or deformed 6-rings (β site), assigning them to the straight and sinusoidal channel walls, respectively.
This study demonstrates that, although spectroscopic techniques accurately identify the local arrangement of Al sites around cationic centres, their location within the periodic framework structure remains unresolved. Our findings underscore the potential of AXRD at the Al K-edge—supported by 27Al MAS NMR and the refined positions of divalent cations used to probe Al pairs—for re-examining the structures of other zeolites exhibiting both short- and long-range order in aluminium distribution.


