Structural basis of the interaction of transcription factors p53 and FOXO4
The teams of Prof. Tomáš Obšil from the Department of Physical and Macromolecular Chemistry, Faculty of Science, Charles University, Doc. Václav Veverka from the Institute of Organic Chemistry and Biochemistry of the CAS and Dr. Veronika Obšilová from the Institute of Physiology of the CAS have elucidated the structural basis of the interaction of key transcription factors p53 and FOXO4. The study was published in the prestigious journal Nature Communications.
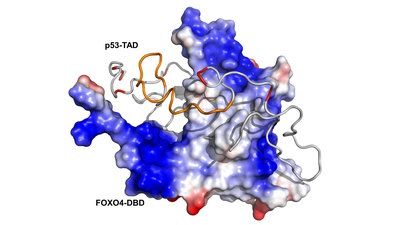
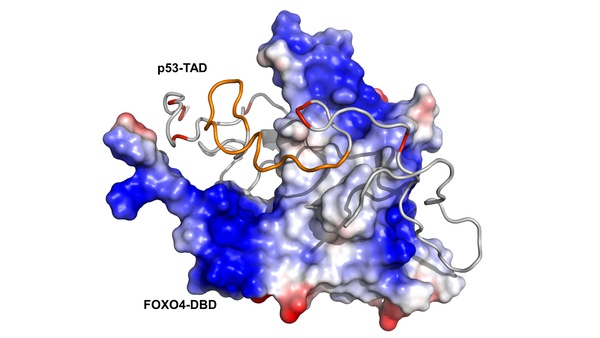
The transcription factors and tumor suppressors FOXO4 and p53 regulate aging, and their deregulation is associated with numerous diseases, including cancer. Under stress conditions, cellular senescence is promoted by p53 sequestration and senescence-associated protein p21 transcriptional upregulation induced by interactions between the FOXO4 Forkhead DNA-binding domain and the p53 transactivation domain. In this study, research teams from the Department of Physical and Macromolecular Chemistry (website), the Institute of Organic Chemistry and Biochemistry of the CAS (website), and the Institute of Physiology of the CAS (website) have demonstrated that the interactions between p53 and FOXO4 exhibit significant heterogeneity. Utilizing nuclear magnetic resonance and molecular modeling, the researchers demonstrated that the transactivation domain of p53 primarily interacts with the N-terminal region of the FOXO4 Forkhead domain, while retaining a high degree of flexibility in the complex. The study further demonstrated that p53 interacts with FOXO4 through multiple binding modes, resulting in substantial structural variability of the complex.
Structural plasticity of the FOXO-DBD:p53-TAD interaction